NSO and the Institute for Safe Medication Practices (ISMP) have teamed up to help you practice safe medication use and keep patients safe. The following is an ISMP Medication Safety Alert from the August 2017 NSO Risk Advisor.
Although
DIASTAT (diaze
PAM rectal gel) has been available for a number of years, the
DIASTAT ACUDIAL delivery system was approved in 2005. The Diastat AcuDial delivery system is used for the acute treatment of patients two years and older on stable anti-epileptic medications who experience bouts of increased seizure activity (both prolonged and cluster seizures). Since the product’s introduction, the Institute for Safe Medications Practices has received a number of reports of errors using the device because it was not properly dialed and locked by the pharmacist prior to dispensing. Some of these errors have led to respiratory depression and required emergent intervention.
In one case, a 4-year-old girl with complex seizure disorder was prescribed Diastat AcuDial 5 mg. The pharmacy, however, dispensed an unlocked 10 mg Diastat AcuDial rectal syringe. Luckily, her parents had been educated to check that the dose was locked in at 5 mg and reported this dispensing mishap to the pediatric neurologist and pharmacy before administering it to their daughter.
A second case involved a young boy weighing 8 kg also with complex partial seizure disorder. Unfortunately, unlike the case above, the parents of this patient were not provided education on what to check for when using Diastat AcuDial. The pharmacy dispensed an unlocked 10 mg Diastat AcuDial rectal syringe and the parents administered the entire contents to their son. The child developed respiratory depression, was transported to the emergency room, and thankfully recovered.
More recently, the parent of a disabled adult with a seizure disorder reported that her daughter received a Diastat AcuDial rectal syringe that had been locked at an incorrect dose. The patient’s prescribed dose was 15 mg, but the pharmacist mistakenly locked the dose at 20 mg. The patient’s parent did not discover the error until after she had administered the dose. The patient was lethargic and slept for over 15 hours following administration.
The Diastat AcuDial delivery system is available as a 10 mg and a 20 mg rectal syringe designed to deliver minimum dosages of 5 mg or 12.5 mg respectively with dosage increments of 2.5 mg. Each package comes with two unlocked rectal syringes per package. Before the product is dispensed, both syringes must be dialed, set, and locked to the prescribed dose by the pharmacist, even when the maximum dose is prescribed. Once set and locked, the prescribed dose will appear in the dose display window and the locking ring, designated with a green ready band, will be engaged. To aid the pharmacist in locking the prescribed dose, the Diastat AcuDial package insert includes pharmacist instructions for the steps that need to be taken before dispensing the product to the patient (
Figure 1; the instructions are also available online at:
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a84a73a7-8e3a-4985-8be0-04b3028f5e49).
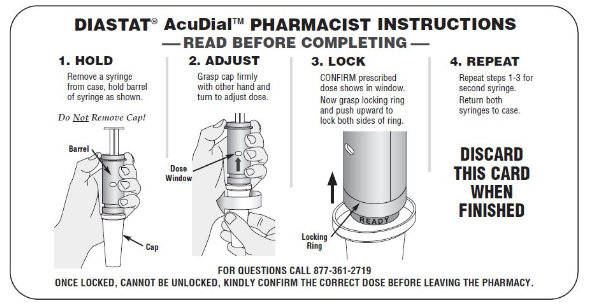 Figure 1. Pharmacist instructions
Figure 1. Pharmacist instructions
Ensure nurses know to confirm the prescribed dose has been dialed, set, and locked by the pharmacist. If the dose has not been locked or the green ready band is not visible, contact the pharmacy prior to administering the medication. Educate patients and caregivers how to use the device, including confirming that: the prescribed dose is visible in the display window; the green ready band is visible; and the smaller rectal tip size is used if the patient is a child. Encourage patients to visit
www.diastat.com for more information about how to administer the medication and create a preparedness plan for family and caregivers to follow.
NSO Risk Advisor is intended to inform Affinity Insurance Services, Inc., customers of potential liability in their practice. It reflects general principles only. It is not intended to offer legal advice or to establish appropriate or acceptable standards of professional conduct. Readers should consult with a lawyer if they have specific concerns. Neither Affinity Insurance Services, Inc., NSO Risk Advisor, nor CNA assumes any liability for how this information is applied in practice or for the accuracy of this information. The professional liability insurance policy is underwritten by American Casualty Company of Reading, Pennsylvania, a CNA company. Coverages, rates and limits may differ or may not be available in all States. All products and services are subject to change without notice. This material is for illustrative purposes only and is not a contract. It is intended to provide a general overview of the products and services offered. Only the policy can provide the actual terms, coverages, amounts, conditions and exclusions. CNA is a service mark and trade name registered with the U.S.Patent and Trademark Office. Nurses Service Organization is a registered trade name of Affinity Insurance Services, Inc.; in CA (License #0795465), MN & OK, AIS Affinity Insurance Agency, Inc.; and in NY, AIS Affinity Insurance Agency. NSO Risk Advisor is published by Affinity Insurance Services, Inc., with headquarters at 1100 Virginia Drive, Suite 250, Fort Washington, PA 19034. Phone: (215) 773-4600.
This publication is intended to inform Affinity Insurance Services, Inc., customers of potential liability in their practice. This information is provided for general informational purposes only and is not intended to provide individualized guidance. All descriptions, summaries or highlights of coverage are for general informational purposes only and do not amend, alter or modify the actual terms or conditions of any insurance policy. Coverage is governed only by the terms and conditions of the relevant policy. Any references to non-Aon, AIS, NSO, HPSO websites are provided solely for convenience, and Aon, AIS, NSO and HPSO disclaims any responsibility with respect to such websites. This information is not intended to offer legal advice or to establish appropriate or acceptable standards of professional conduct. Readers should consult with a lawyer if they have specific concerns. Neither Affinity Insurance Services, Inc., NSO, nor CNA assumes any liability for how this information is applied in practice or for the accuracy of this information.
Nurses Service Organization is a registered trade name of Affinity Insurance Services, Inc., a licensed producer in all states (TX 13695); (AR 100106022); in CA, MN, AIS Affinity Insurance Agency, Inc. (CA 0795465); in OK, AIS Affinity Insurance Services, Inc.; in CA, Aon Affinity Insurance Services, Inc., (CA 0G94493), Aon Direct Insurance Administrators and Berkely Insurance Agency and in NY, AIS Affinity Insurance Agency.